Aseptic pharmaceutical manufacturing is a highly controlled process designed to ensure that sterile products are free from microbial contamination. Environmental monitoring (EM) is a key component of this process, aimed at ensuring the cleanliness and sterility of the manufacturing environment. Effective environmental monitoring is critical to maintaining product quality and complying with regulatory requirements set by authorities such as the EU Annex 1 guidelines.
The aseptic process involves filling and packaging sterile products in an environment that must remain free from contamination. Environmental monitoring helps detect and quantify airborne and surface-based microbial and particulate contamination in critical areas such as cleanrooms and sterile suites. An Environmental Monitoring System (EMS) is made up of a system of filters and sensors that connect to a processing software. It should alert you when designated environmental factors go outside of normal limits.
One of the most important aspects of environmental monitoring is the assessment of airborne particles. These particles can be categorized into two categories, non-viable and viable particles. Non-viable particles are inanimate particles such as dust, fibres, and skin cells that can carry viable microorganisms and potentially lead to contamination. They are measured by particle counters that detect different sizes of airborne particles, helping to maintain a particle-free environment. Viable particles refer to microorganisms like bacteria and fungi that are present in the air. Active and passive air sampling techniques are used to capture these particles for microbial testing. Regular air sampling in critical areas ensures that the environment does not foster microbial contamination. Annex 1 has the limits for airborne particulate concentration for non-viable particulates published, with the limits for each cleanroom classification identified.

Non-viable airborne particles can be measured by laser particle counters that draw air into the device, where a laser beam illuminates the particles, and a sensor counts them based on size (typically in microns). A real-time monitoring system continuously monitors non-viable particle levels in cleanrooms and provides real-time feedback. They can trigger alarms if particle counts exceed specified limits, allowing for immediate corrective actions.
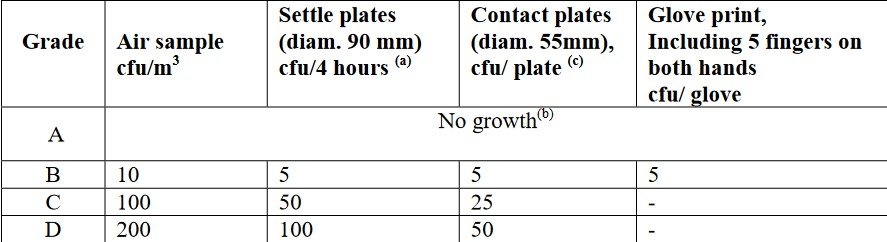
Viable airborne particles can be monitored by active air sampling where air is actively drawn into a sampler device that captures airborne microorganisms on an agar plate or filter. Common devices used for active air sampling include impactors or slit-to-agar samplers, which pull a known volume of air over a culture medium. After sampling, the agar plates are incubated to allow any viable microorganisms to grow, which are then counted as colony-forming units (CFU). Passive Air Sampling using Settle Plates consists of open agar plates that are left exposed to the environment for a specified period to allow microorganisms to settle on the surface. This method is less quantitative but provides a broad indication of the viable contamination load in the air. Airborne Particle Samplers can collect microorganisms on filters or growth media to determine the microbial load in the air. The collected samples are incubated, and the colonies are counted to evaluate the contamination levels. This table shows Annex 1 guidelines for maximum action limits for viable particle contamination, with the limits for each cleanroom classification identified.
MesaLabs is a Bronze Sponsor of our upcoming summit on aseptic processing and is a supplier of monitoring equipment and solutions that can be utilised to adhere to Annex 1 guidelines to ensure that airborne contamination is prevented in the final product. Mesa Labs makes it possible to monitor particulate matter and environmental conditions for EPA compliance with convenient, reliable solutions manufactured in our NIST-traceable and ISO-9001:2015 certified facilities. They offer a solution portfolio that includes ambient air samplers, environmental cyclones and air flow calibrators as well as equipment services for repair and calibration.
Environmental monitoring is a vital part of aseptic pharmaceutical manufacturing. By carefully monitoring airborne particles, surface contamination, and environmental parameters, manufacturers can maintain the integrity of their sterile products and ensure compliance with regulatory standards. Effective environmental monitoring reduces the risk of contamination, safeguarding product quality and patient safety. To meet with MesaLabs and many other solution providers and esteemed speakers from across the industry at the Innovatrix ‘3rd Aseptic BioPharma Processing Summit’ in Vienna, Austria, on October 29–30, 2024.
For more information, visit our website or email us at info@innovatrix.eu for the event agenda.